
Cork, 15th May, 2024. May marks Cystic Fibrosis (CF) Awareness Month and this year’s focused theme is Resilient. CF is a genetic disease which affects the lungs and the digestive system and is caused by mutations in the CFFTR gene which leads to the production of a thick sticky mucus in the lungs and it can also affect other organs. According to the Cystic Fibrosis Foundation approximately 1,000 new cases of CF are diagnosed each year. More than 75 percent of people with CF are diagnosed by age 2. More than half of the CF population is age 18 or older.
Resilience certainly applies to Dr. Michael Twomey a Cork native who is living with CF. Michael as well as being the founder of the I Know Me app also works as a Senior Clinical Research Manager at Health Innovation Hub Ireland which connects innovators with healthcare. (pictured) Michael’s own research and innovation journey is a very personal one, motivated by the death of his sister from CF in 1997. Based on his own life and patient journey he strove to address the problem faced by fellow CF patients and carers, the challenge of recalling medical history at medical appointments. He was concerned about providing accurate information to his own clinicians and he decided to do something about it, as poor quality information imparted by patients or their carers is known to affect patient outcomes.
For patients with CF, there are a number of medical appointments necessary to manage the disease and improve or maintain the CF’s patient’s condition. The aim of the appointment is to arrive at correct diagnosis and make decisions regarding appropriate treatment plans. One of the key data facets required by the clinician in the diagnostic process is accurate medical history supplied by the patient or carer. This information is a complex tapestry of illnesses, medications, symptoms and procedures peppered with medical appointments. which can be difficult to recall accurately. Memory recall requires the patient to pull data from their long-term declarative memory, a process that is made more difficult by the nature of the medical encounter itself, which can be stressful and require a medical literacy that many struggle with.
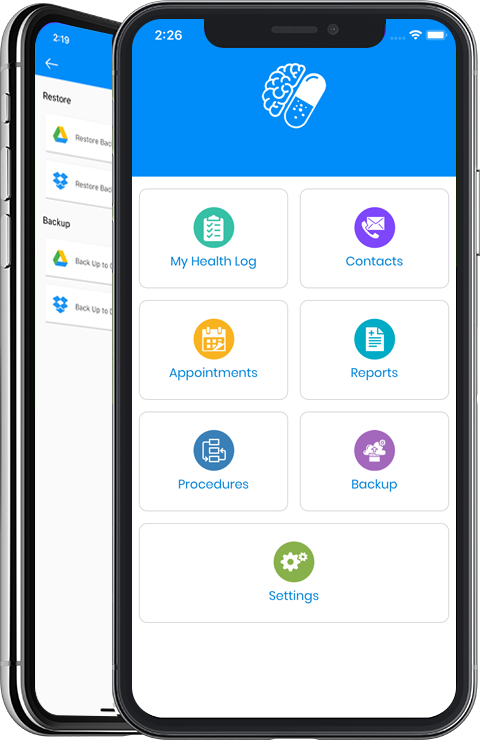
With this in mind, Michael came up a number of solutions to this problem, first a paper-based checklist booklet which was sent out to every CF patient in Ireland, and then following the learning from same he designed and developed the I Know Me app. This was again done with the help of fellow CF patients and caregivers which basically means it’s designed by CF patients or carers for CF patients or carers. The app was specifically built and evaluated to address the above problem of poor CF patient/carer memory recall. The I Know Me design is based on current CF medical appointment workflows, CF patient / carer experiences, and helps the patient/carer record, track and report on key aspects of their medical history. These reports can then be shared with a patient’s clinical team if and when required. Additionally, the app allows for the capture of multiple health profiles on one device, where switching between profiles is easy. This component of the app is vital for families with more than one CF patient.
Some of the app features are the ability to provide the CF patient with a valuable health management tool, tracking symptoms, key metrics, vaccines, nutrition, physiotherapy, medications and other treatments and procedures (including the results of same). Furthermore, it allows patient and carers to bring their or their child’s health autobiography with them to the medical appointment, reducing the stress of having to recall and providing the clinician with better quality data. Not only that it also allows for the capture of data regarding what happened within the medical appointment itself, this is vital later when the patient or carer need to remember therapies, procedures etc. Not surprising then that this information will aid adherence to therapies. But possibly the most interesting feature of the app is that it generates reports based on metrics which allows the CF patient to track changes and development in health status over time. The portability and accessibility of the solution not only aids memory recall it also empowers patients and carers giving them peace of mind when they travel (as they now have their medical histories with them) and go about their busy lives.
Today, Health Innovation Hub Ireland launched the results of the pilot study of this digital health app which is set to be a gamechanger for people living with CF as it now enables patients to manage their medical history more effectively. HIHI conducted a pilot of the I Know Me app with 15 CF patients/carers within Ireland, including the facilitation of a number of design workshops which helped offer a dynamic and inclusive approach to problem solving, enabling participants to create a solution that is not only functional, but also deeply resonant with CF patients/carers. HIHI analysed the pilot results (end-user feedback) and prepared a final project report to disseminate the outcome of the pilot. The project was granted ethical approval by the SREC in University College Cork.
When tested with patients and carers during the pilot it was agreed that the app is an essential tool for CF patients with 93% of users saying that it improved memory recall. Additionally, it was found that:
93% Agreed or Strongly Agreed that the app was easy to navigate
87% Agreed or Strongly Agreed that using the app requires a change in behaviour
86% Agreed or Strongly Agreed that the app was easy to use
67% stating that it was Very or Extremely useful for memory recall of medical history.
67% Agreed or Strongly Agreed that using the app gave them a real sense of empowerment
67% of participants use the app on a weekly or monthly basis
60% Agreed or Strongly Agreed that using the app helped reduce their stress levels within the medical appointment.
The pilot results will come as welcome news to CF patients as in Ireland, there are almost 1,700 Irish people living with the disease and 70,000 CF patients worldwide.
Speaking on the release of HIHI’s I Know Me pilot study today, Dr. Michael Twomey the founder of app said: “I Know Me is an app for us (CF patients / carers) designed by us, that’s why it works! It allows us to track our medications, our symptoms etc more effectively, our own unique health journeys, with the data captured available to us as required. We are empowered in the management of our own health and need to be. After-all, we need to be the CEOs of our own bodies! I’m delighted that we are making such progress, the insights gained from the pilot have been invaluable and will really aid our advancement of the I Know Me app. Still more work to be done, I hope my sister (who passed away with CF in 1997) would be proud of what we have done so far.”
Praise from one of the CF patients on the pilot “I really like the way the app gives you visuals on key metrics and allows one to generate reports that can be sent / given to the clinical team. It’s wonderful to be able to refer to the app with confidence re my medical history”
Read the full case study here I Know Me (hih.ie)
The I Know Me app is free and is available on Google Play and can also be downloaded from the Apple App store.
Details of the app can be found on this link https://iknowme.info/












