Irish company Ostoform provides solutions for patients post-surgery with their medical device® Mouldable Seal with FLOWASSIST®which is now available for public and private patients in Ireland
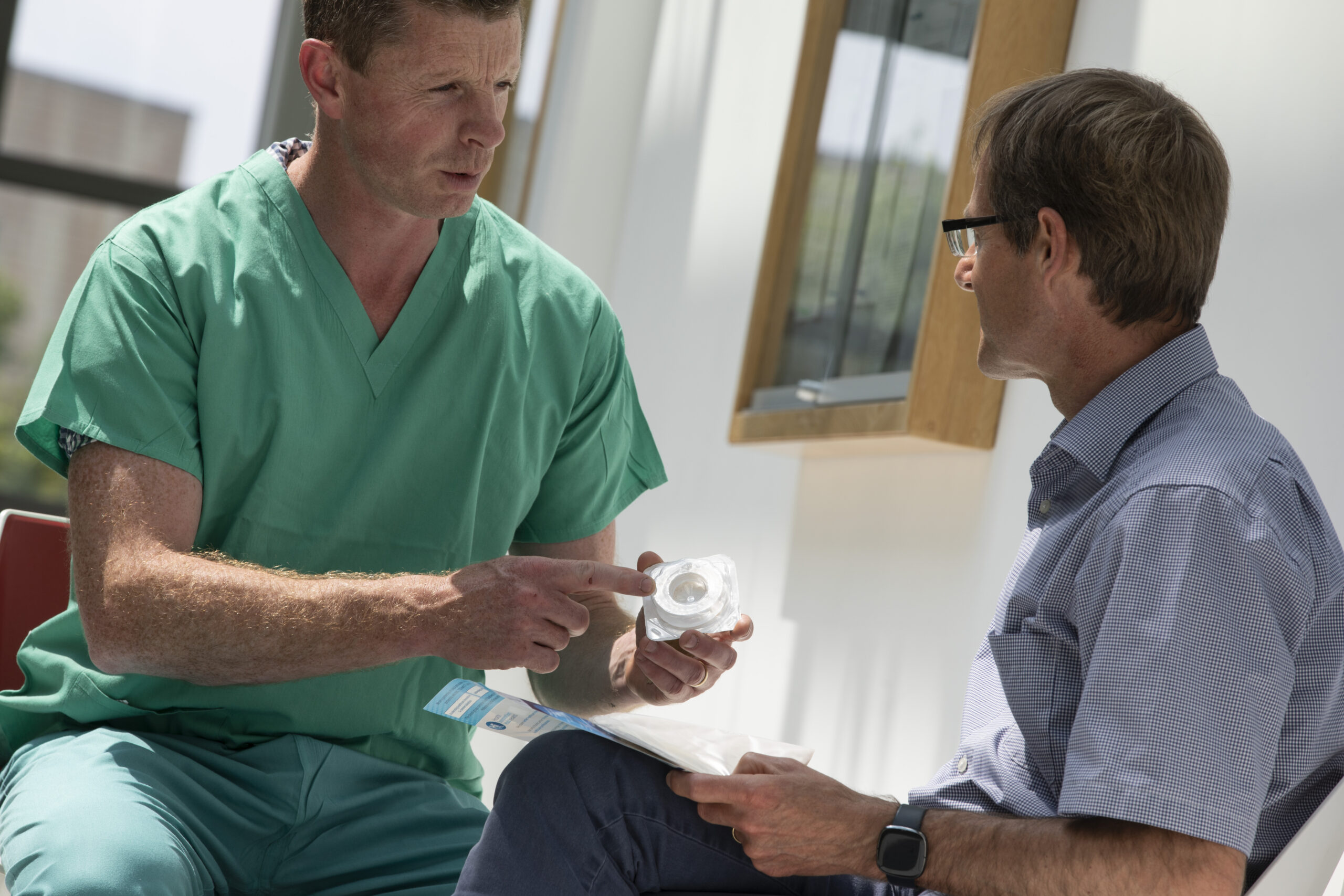
Health Innovation Hub Ireland worked with Ostoform on a product pilot which will change the lives of Irish patients post -surgery.
HIHI along with Kevin Kelleher, CEO of Ostoform, worked on a healthcare challenge. People who have had a surgically created stoma, particularly an ileostomy, are at risk of developing peristomal skin complications, with incidence rates of up to 63% reported in literature. Currently, treatments of peristomal skin complications are limited. Pastes are available, but they can often impede ostomy bags from sticking to the skin. Furthermore, the bags themselves can contribute to peristomal skin complications, by allowing waste to contact the peristomal skin, causing chemical irritation and standard seals are often broken down by stoma output, which then contacts your skin resulting in irritation and discomfort.
The Healthcare Solution is the Ostoform Class I medical device aims to prevent the development of peristomal skin complications. With all ileostomies, there is a risk of stoma output contacting the peristomal skin and causing irritation. Ostoform was designed to minimise this risk. The Ostoform Seal is a device designed to prevent the development of peristomal skin complications and ensures that stoma output flows away from the skin, keeping it safe, healthy and comfortable.
HIHI played a pivotal role in the development of this product pilot and designed and project managed two separate multisite clinical studies to assess the impact and efficacy of their FLOWASSIST novel ostomy device. The initial study included Tallaght University Hospital St James’s Hospital and University Hospital Galway, engaging two CRFs in Dublin and Galway.
The second study was conducted at Cork University Hospital, Mercy University Hospital Cork and University Hospital Galway. Patients with an ileostomy were screened from the stoma nurse’s outpatients list, those who met the inclusion criteria were invited to participate. There was no control group in this study as currently there is no accepted ‘standard care’ device for the management of peristomal skin complications so patients use a range of different devices. Ethics approval was obtained for both studies.
The outcome of the product pilot obtained its objectives. The purpose of this practical application study was to evaluate the effectiveness of the Ostoform® Mouldable Seal with FLOWASSIST® Protection in protecting the skin of people with an ileostomy, as well as to gather user feedback on the perception of the device. 60% of the participants remained at a very low DET score throughout the study, and 35% of participants demonstrated an improvement in DET score provides an indication that the novel barrier ring can be effective in protecting the skin. User feedback was positive with respect to comfort, device handling and the perception of the device’s ability to protect the skin. Furthermore, the majority of participants who already used a barrier ring, indicated that the FLOWASSIST device would result in a longer wear time.
In 2021, the clinical study with Ostoform and HIHI concluded and results were accepted for publication. An application for inclusion of this product on HSE Primary Care Reimbursement Scheme for public patients was submitted to the HSE and approved in September 2022. As a result, the Ostoform FLOWASSIST product is now available to Irish patients.
Kevin Kelleher Ostoform’s CEO spoke about the product pilot and HIHI’s involvement. “HIHI supported Ostoform by preparing and conducting two separate clinical studies, both of which demonstrated very encouraing results. Our FLOWASSIST technology demonstrated a statistically significant reduction in peristomal skin complications, along with additional user benefits. As a result, the product has been awarded premium reimbursement by the HSE. The HIHI played a central role in getting the Ostoform Seal to market in Ireland, and this will result in improved quality of life for ostomy patients across the country”.
Ms Noreen Lynch, Health Innovation Hub Ireland Clinical Liaison said: “As a nurse, I am always looking for new products that make it easier for patients to manage their recovery after surgery. We were delighted to support Ostoform to evaluate their FlowAssist seal with ileostomy patients and to see the real impact these products have on patients. The product is now available in Ireland to public and private patients and is already on the international market”.
Read the full case study here – https://hih.ie/downloads/case-studies/HIHI-case-study_Ostoform-Mar-2023.pdf




