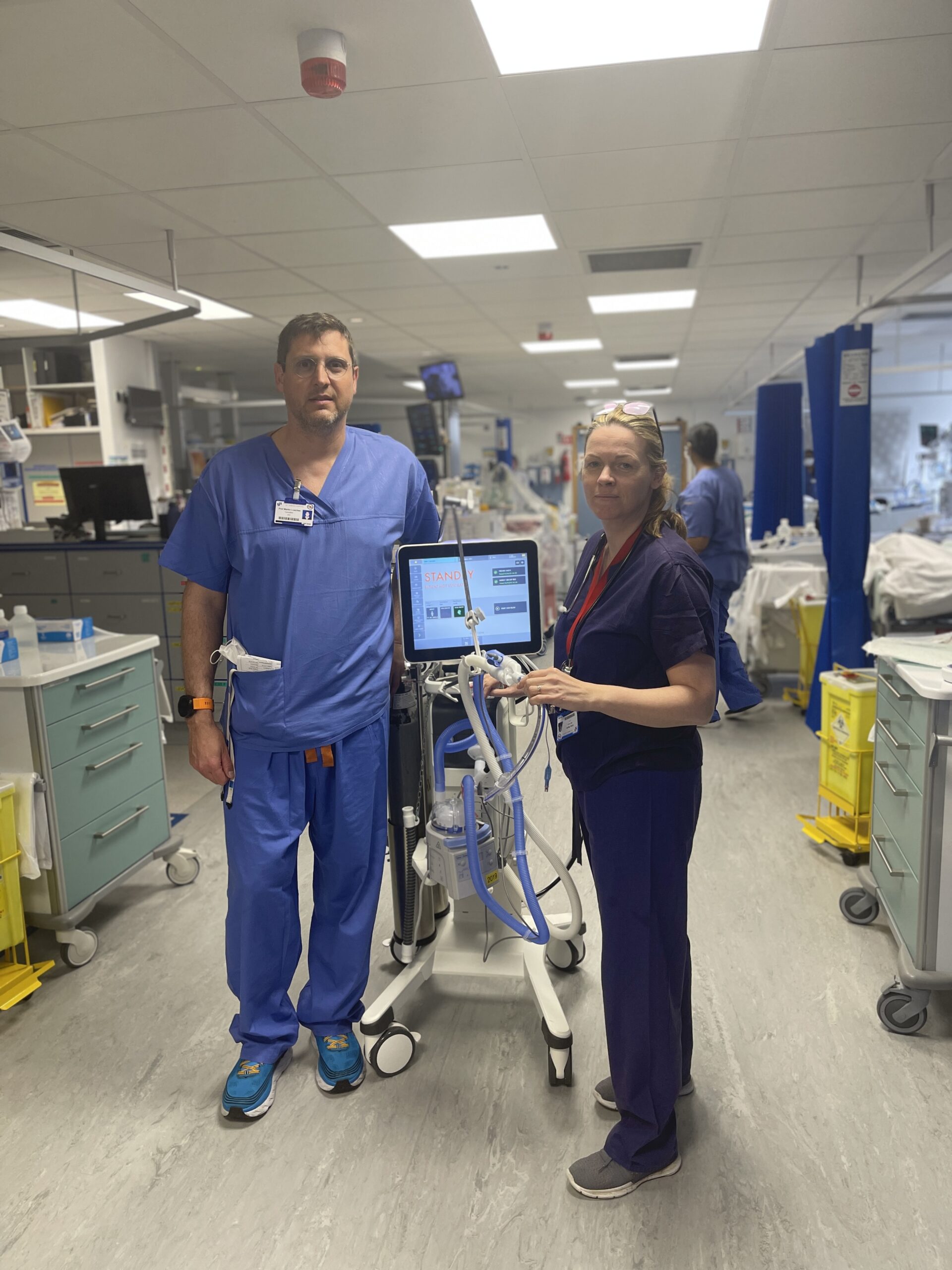
ICU Team develop new healthcare solution that will change the lives of mechanically ventilated patients
Pictured left to right Consultant intensivist and Professor of Medicine in TCD Prof Ignacio Martin-Loeches and Emily Naylor Clinical Nurse Facilitator, St. James’s Hospital
5th April, 2023. Enterprise Ireland through Health Innovation Hub Ireland (HIHI) and in collaboration with Trinity College Dublin (TCD), is supporting a team from St. James’s Hospital Dublin, to assess the commercial feasibility of a novel medical device for tackling Ventilator-associated Pneumonia (VAP). The team, consisting of two intensive care unit nurses (Emily Naylor and Beatriz Tejada Rios) and a consultant intensivist and Professor of Medicine in TCD (Prof Ignacio Martin-Loeches) who deal with the problem of VAP on a daily basis, have developed a novel and innovative solution to tackle this serious medical condition. The feasibility study will assess and map out the route to market for their product, with the ultimate aim of getting the product into the hands of clinical teams treating ventilated patients.
VAP is a serious healthcare problem that affects many ventilated patients with a high mortality rate and is associated with significant health and economic costs. The incidence rate of VAP is a common complication among mechanically ventilated patients in intensive care units, with some studies reporting an incidence rate ranging from 5% to 30%. The total costs of VAP can include direct costs such as hospitalization, laboratory tests, and antibiotics and indirect costs such as lost productivity, decreased quality of life, and mortality. The cost of VAP has been reported to be substantial, with some estimates indicating that it can range from €40,000 to €50,000 per episode.
Emily and Beatriz identified VAP as a healthcare problem that could be minimised through innovative technology, along with Prof. Martin-Loeches, the team, worked with HIHI, a medical device designer to develop the concept. This was reviewed by TCD’s Technology Transfer Office for patentability, allowing for a patent to be filed on the design. The team will work with Associate Prof. Eduardo Ruiz-Hernandez, TCD, to develop a prototype.
With the patent on the way and a solid solution direction identified, Enterprise Ireland has supported these clinical innovators via their Commercial Case Feasibility grant, recognizing the potential of their solution to address this life-threatening condition in a meaningful way.
This project is a great example of the Irish clinical, academic, and government systems working together to support frontline healthcare innovators and improve patient outcomes.
Dr Steven Griffin, Clinical Innovation Manager, Health Innovation Hub Ireland welcomed this new solution to VAP.
“At Health Innovation Hub Ireland, we are committed to fostering innovation in healthcare and supporting the development of early-stage ideas into viable products. Emily Naylor previously worked with HIHI before returning to clinical practice in ICU Department of St James’- I like to think our innovation process rubbed off on her, but the reality is that frontline staff understand the problems better than anyone and between them the team at St James’ were driven to develop a solution to VAP. The collaboration with St. James’s Hospital Dublin, Trinity College Dublin, and Enterprise Ireland on the development of a novel solution to tackle Ventilator-associated Pneumonia (VAP) is a prime example of how Health Innovation Hub Ireland can support innovative thinking and bring positive change to the healthcare system. We are excited to see the potential impact this project can have on patient outcomes and the healthcare industry as a whole.”
Emily Naylor, Clinical Nurse Facilitator, St. James’s Hospital commented that: “The support from Enterprise Ireland is crucial in our efforts to tackle Ventilator-associated Pneumonia, a serious medical condition affecting many patients. Our team, in collaboration with the Health Innovation Hub Ireland and Trinity College Dublin, have developed a novel solution to address this issue and bring positive change to patient outcomes. With Enterprise Ireland’s funding and resources, we are confident in our ability to bring our innovative product to market and make a meaningful impact for patients, carers and the healthcare system”.
About HIHI
Health Innovation Hub Ireland (HIHI) drives collaboration between the health service and enterprise. We offer companies the opportunity to pilot and/or participate in clinical evaluation studies to prove their products. We provide the health service access to innovative products, services and devices that they may not otherwise be exposed to. HIHI is built on the recognition that collaboration with enterprise can benefit patient care, patient pathways and outcomes. We assess all concepts for healthcare innovation from those on the frontline. We encourage healthcare professionals to get in touch with HIHI if they have an idea or solution to how something in your job might work better. HIHI was established by the Department of Business, Enterprise and Innovation and the Department of Health and is supported by Enterprise Ireland (EI) and the Health Service Executive (HSE).
About TCD – Technology Transfer Team at Trinity Innovation
The Technology Transfer Team at Trinity Innovation supports and enables the research community to translate innovative research excellence into outputs for maximum societal and economic impact. Commercialisation, intellectual property protection and knowledge transfer is an integral component of the technology transfer journey and is supported by Trinity Innovation. The team at Trinity are actively nurturing alliances with Hospital-based innovation initiatives – this project is a great example of a group of clinical professionals with an idea for a health innovation, connecting with Trinity Innovation resources to move the project forward in partnership
About EI
Enterprise Ireland is the Irish government agency responsible for the development and growth of Irish enterprises in world markets. They provide supports for early-stage innovations by funding, mentoring, and advising companies through their various programs. They offer a wide range of financial supports such as seed capital, venture capital, and equity funding for startups, as well as funding for product development and commercialization. Enterprise Ireland also provides access to a network of industry experts, mentors, and partners to help companies develop their products and enter international markets. They also provide resources and training to help entrepreneurs develop the necessary skills and knowledge to grow their businesses. Their goal is to help Irish companies to start, scale and succeed in global markets.





:quality(70)/cloudfront-eu-central-1.images.arcpublishing.com/irishtimes/DXVHIAGDUJGYPP34EYR2UNZTDA.jpg)

